Gcse c5 electrolysis revison
•
2 j'aime•1,281 vues
Signaler
Partager
Signaler
Partager
Recommandé
Recommandé
Contenu connexe
Tendances
Tendances (20)
En vedette (12)
C:\Fakepath\Notes Ii Standard Molar Volume And Iii The Ideal Gas Law

C:\Fakepath\Notes Ii Standard Molar Volume And Iii The Ideal Gas Law
Plus de Linton Village College
Plus de Linton Village College (20)
Gcse c4 chemical patterns bonding & periodic table revision

Gcse c4 chemical patterns bonding & periodic table revision
Dernier
Mehran University Newsletter Vol-X, Issue-I, 2024

Mehran University Newsletter Vol-X, Issue-I, 2024Mehran University of Engineering & Technology, Jamshoro
Dernier (20)
Unit-IV; Professional Sales Representative (PSR).pptx

Unit-IV; Professional Sales Representative (PSR).pptx
This PowerPoint helps students to consider the concept of infinity.

This PowerPoint helps students to consider the concept of infinity.
Unit-V; Pricing (Pharma Marketing Management).pptx

Unit-V; Pricing (Pharma Marketing Management).pptx
Basic Civil Engineering first year Notes- Chapter 4 Building.pptx

Basic Civil Engineering first year Notes- Chapter 4 Building.pptx
Python Notes for mca i year students osmania university.docx

Python Notes for mca i year students osmania university.docx
ICT role in 21st century education and it's challenges.

ICT role in 21st century education and it's challenges.
General Principles of Intellectual Property: Concepts of Intellectual Proper...

General Principles of Intellectual Property: Concepts of Intellectual Proper...
Micro-Scholarship, What it is, How can it help me.pdf

Micro-Scholarship, What it is, How can it help me.pdf
Seal of Good Local Governance (SGLG) 2024Final.pptx

Seal of Good Local Governance (SGLG) 2024Final.pptx
Asian American Pacific Islander Month DDSD 2024.pptx

Asian American Pacific Islander Month DDSD 2024.pptx
Gcse c5 electrolysis revison
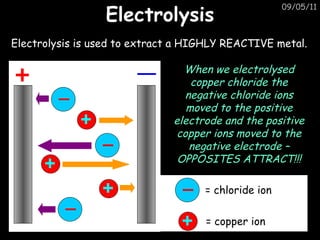
- 1. Electrolysis 09/05/11 Electrolysis is used to extract a HIGHLY REACTIVE metal. When we electrolysed copper chloride the negative chloride ions moved to the positive electrode and the positive copper ions moved to the negative electrode – OPPOSITES ATTRACT!!! = chloride ion = copper ion
- 2. Electrolysis equations 09/05/11 We need to be able to write “half equations” to show what happens during electrolysis (e.g. for copper chloride): 2 2 2 At the negative electrode the positive ions GAIN electrons to become neutral copper ATOMS. The half equation is: Cu 2+ + e - Cu At the positive electrode the negative ions LOSE electrons to become neutral chlorine MOLECULES. The half equation is: Cl - - e - Cl 2
