EAS
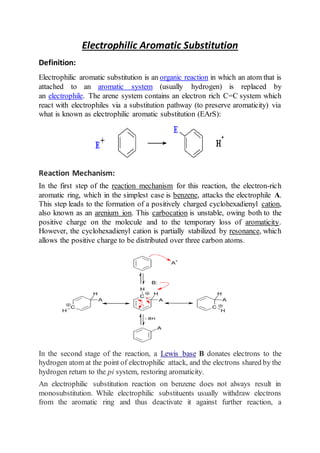
- 1. Electrophilic Aromatic Substitution Definition: Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. The arene system contains an electron rich C=C system which react with electrophiles via a substitution pathway (to preserve aromaticity) via what is known as electrophilic aromatic substitution (EArS): Reaction Mechanism: In the first step of the reaction mechanism for this reaction, the electron-rich aromatic ring, which in the simplest case is benzene, attacks the electrophile A. This step leads to the formation of a positively charged cyclohexadienyl cation, also known as an arenium ion. This carbocation is unstable, owing both to the positive charge on the molecule and to the temporary loss of aromaticity. However, the cyclohexadienyl cation is partially stabilized by resonance, which allows the positive charge to be distributed over three carbon atoms. In the second stage of the reaction, a Lewis base B donates electrons to the hydrogen atom at the point of electrophilic attack, and the electrons shared by the hydrogen return to the pi system, restoring aromaticity. An electrophilic substitution reaction on benzene does not always result in monosubstitution. While electrophilic substituents usually withdraw electrons from the aromatic ring and thus deactivate it against further reaction, a
- 2. sufficiently strong electrophile can perform a second or even a third substitution. This is especially the case with the use of catalysts. Examples Table of EAS: Reaction Reagents Electrophile Product Comments Nitration HNO3 / H2SO4 NO2+ E+ formed by loss of water from nitric acid Sulfonation H2SO4 or SO3 / H2SO4 SO3 Reversible Halogenation Cl2 / Fe or FeCl3 Cl+ E+ formed by Lewis acid removing Cl- Br2 / Fe or FeBr3 Br+ E+ formed by Lewis acid removing Br- Alkylation R-Cl / AlCl3 R+ E+ formed by Lewis acid removing Cl- R-OH / H+ R+ E+ formed by loss of water from alcohol C=C / H+ R+ E+ formed by protonation of alkene Acylation RCOCl / AlCl3 RCO+ E+ formed by Lewis acid removing Cl- RCO2COR / AlCl3 RCO+ E+ formed by Lewis acid removing RCO2-
- 3. Examples of electrophilic aromatic substitution: Nitration of Benzene: The sourceof the nitronium ion is through the protonation of nitric acid by sulfuric acid, which causes the loss of a water molecule and formation of a nitronium ion. Sulfuric Acid Activation of Nitric Acid The first step in the nitration of benzene is to activate HNO3with sulfuric acid to producea stronger electrophile, the nitronium ion. Because the nitronium ion is a good electrophile, it is attacked by benzene to produceNitrobenzene. Mechanism (Resonance forms of the intermediate can be seen in the generalized electrophilic aromatic substitution)
- 4. Sulfonation of Benzene: Sulfonation is a reversible reaction that produces benzenesulfonic acid by adding sulfur trioxide and fuming sulfuric acid. The reaction is reversed by adding hot aqueous acid to benzenesulfonic acid to producebenzene. Mechanism To producebenzenesulfonic acid from benzene, fuming sulfuric acid and sulfur trioxide are added. Fuming sulfuric acid, also refered to asoleum, is a concentrated solution of dissolved sulfur trioxide in sulfuric acid. The sulfur in sulfur trioxide is electrophilic because the oxygens pull electrons away from it because oxygen is very electronegative. The benzene attacks the sulfur (and subsequent protontransfers occur)to producebenzenesulfonic acid. Halogenation of Benzene:
- 5. MECHANISM FOR HALOGENATION OF BENZENE Step 1: The bromine reacts with the Lewis acid to form a complex that makes the bromine more electrophilic. Step 2: The π electrons of the aromatic C=C act as a nucleophile, attacking the electrophilic Br, and displacing iron tetrabromide. This step destroys the aromaticity giving the cyclohexadienyl cation intermediate. Step 3: Removal of the protonfrom the sp3 C bearing the bromo - group reforms the C=C the aromatic system, generating HBr and regenerating the active catalyst. Orientation: The rate data shows that toluene is more reactive or activated with respect to benzeneand the productdistribution shows that the methyl group directs the new substituent to the ortho- and para- positions In contrast, trifluoromethylbenzene is less reactive or deactivatedwith respect to benzene and directs the new substituent to the meta position. Here is a table that shows the effect of substituents on a benzene ring have on both the rate and orientation of electrophilic aromatic substitution reactions.
- 6. There are two main electronic effects that substituents can exert: RESONANCE : Resonance effects are those that occurthrough the system and can be represented by resonance structures. These can be either electron donating (e.g. -OMe) where electrons are pushed toward the arene or electron withdrawing (e.g. -C=O) where electrons are drawn away from the arene. INDUCTIVE :
- 7. Inductive effects are those that occurthrough the system due to electronegativity type effects. These too can be either electron donating (e.g. - Me) where electrons are pushed toward the arene or electron withdrawing (e.g. -CF3, +NR3) where electrons are drawn away from the arene. A simplified approachto understanding substituent effects is given here, based on the "isolated molecule approach". The text uses the more rigorous approach of drawing the resonance structures for the intermediate formed by attack at each of the o-, m- and p- positions. Ortho/para directors Groups with unshared pairs of electrons, suchas the amino group of aniline, are strongly activating and ortho/para-directing. Such activating groups donate those unshared electrons to the pi system. When the electrophile attacks the ortho and para positions of aniline, the nitrogen atom can donate electron density to the pi system (forming an iminium ion), giving four resonance structures (as opposedto three in the basic reaction). This substantially enhances the stability of the cationic intermediate.
- 8. Compare this with the case when the electrophile attacks the meta position. In that case, the nitrogen atom cannot donate electron density to the pi system, giving only three resonance contributors. For this reason, the meta-substituted productis produced in much smaller proportion to the ortho and para products. Other substituents, such as the alkyl and aryl substituents, may also donate electron density to the pi system; however, since they lack an available unshared pair of electrons, their ability to do this is rather limited. Thus, they only weakly activate the ring and do not strongly disfavor the meta position. Halogens are ortho/para directors, since they possessan unshared pair of electrons just as nitrogen does. However, the stability this provides is offset by the fact that halogens are substantially more electronegative than carbon, and thus draw electron density away from the pi system. This destabilizes the cationic intermediate, and EAS occurs less readily. Halogens are therefore deactivating groups. Directed ortho metalation is a special type of EAS with special ortho directors. Meta directors: Non-halogen groups with atoms that are more electronegative than carbon, such as a carboxylic acid group (CO2H) draw substantial electron density from the pi system. These groups are strongly deactivating groups. Additionally, since the substituted carbonis already electron-poor, the resonance contributor with a positive charge on this carbon (produced by ortho/para attack) is less stable than the others. Therefore, these electron-withdrawing groups are meta directing.
- 9. Reaction mechanism: In the first step of the reaction mechanism for this reaction, the electron-rich aromatic ring, which in the simplest case is benzene, attacks the electrophile A. This step leads to the formation of a positively charged cyclohexadienyl cation, also known as an arenium ion. This carbocation is unstable, owing both to the positive charge on the molecule and to the temporary loss of aromaticity. However, the cyclohexadienyl cation is partially stabilized by resonance, which allows the positive charge to be distributed over three carbon atoms. In the second stageofthe reaction, a Lewis baseB donates electrons to the hydrogen atom at the point of electrophilic attack, and the electrons shared by the hydrogen return to the pi system, restoring aromaticity. An electrophilic substitution reaction on benzene does not always result in monosubstitution. While electrophilic substituents usually withdraw electrons from the aromatic ring and thus deactivate it against further reaction, a sufficiently strong electrophile can perform a second oreven a third substitution. This is especially the case with the use of catalysts. Five membered heterocyclic compunds: Furan, thiophene, pyrrole and their derivatives are all highly activated compared to benzene. These compounds all contain an atom with an unshared pair of electrons (oxygen,sulphur, or nitrogen) as a member of the aromatic ring, which substantially increases the stability of the cationic intermediate. Examples of electrophilic substitutions to pyrrole are the Pictet–Spengler reaction and the Bischler–Napieralski reaction.